Titanium Anode Lifespan Testing: How to Accurately Predict Service Life

Table of Contents
- The Importance of Titanium Anode Lifespan Testing in Industrial Applications
- Understanding Failure Mechanisms: Passivation and Coating Consumption
- The Accelerated Life Test (ALT): Methodology and Standards
- Key Parameters in ALT: Current Density and Electrolyte Concentration
- Visual and Microscopic Analysis: Monitoring Surface Morphology Changes
- Voltage-Time Curves: Identifying the Onset of Anode Passivation
- From Lab to Field: How to Extrapolate Real-World Service Life
- Factors Influencing Testing Accuracy: Temperature and Impurities
- Integrating Testing into Predictive Maintenance for Electrolytic Systems
- Selecting the Correct Anode Coating to Maximize Operational Longevity
The Importance of Titanium Anode Lifespan Testing in Industrial Applications
In industrial electrochemical processes—ranging from sodium hypochlorite generation to electroplating and electrowinning—the titanium anode is a critical consumable. Its performance directly dictates the energy efficiency and output quality of the entire system. However, unlike mechanical components, the degradation of a Mixed Metal Oxide (MMO) or platinum-coated anode is often invisible to the naked eye until a catastrophic failure occurs.
Lifespan testing is not merely a quality control metric; it is a vital technical requirement for several reasons:
- Operational Reliability: Unexpected anode failure can lead to unplanned downtime, which is costly in high-volume manufacturing environments.
- Energy Efficiency: As coatings deplete, the overpotential increases. Testing helps identify the point where the voltage-to-yield ratio becomes economically unviable.
- Risk Mitigation: In applications like cathodic protection or wastewater treatment, knowing the remaining service life ensures environmental and structural safety compliance.
- Material Validation: It allows engineers to verify if the coating thickness and composition (e.g., Ruthenium-Iridium vs. Iridium-Tantalum) match the specific chemical demands of their electrolyte.
By implementing standardized lifespan testing, facilities can transition from reactive replacement to proactive maintenance, ensuring that the titanium anodes operate within their optimal electrochemical window for as long as possible.
Understanding Failure Mechanisms: Passivation and Coating Consumption
To accurately predict service life, one must first understand how a titanium anode fails. In most industrial environments, the degradation of Mixed Metal Oxide (MMO) anodes typically follows two distinct technical paths: physical consumption of the electrocatalytic layer and the formation of a non-conductive interface.

1. Electrocatalytic Coating ConsumptionDuring electrolysis, the precious metal oxides (such as RuO2 or IrO2) undergo slow dissolution or wear. As the current flows, the active sites on the coating surface are gradually exhausted. Once the coating thickness reaches a critical minimum, the underlying titanium substrate is exposed to the electrolyte, leading to a sharp rise in cell voltage.
2. Titanium Substrate PassivationThis occurs when the electrolyte penetrates the coating pores and reaches the titanium base. Under anodic polarization, the titanium reacts with oxygen to form a thin, insulating layer of Titanium Dioxide (TiO2). This "passivation layer" blocks electron transfer even if some active coating remains on the surface. This is common in applications with high oxygen evolution or poor coating adhesion.
- Chemical Attack: Specific ions (like Fluoride) can dissolve the protective TiO2 layer, accelerating substrate corrosion.
- Mechanical Spalling: High gas evolution rates or physical abrasion can strip the coating from the substrate prematurely.
Understanding whether an anode is failing due to uniform consumption or localized passivation is essential for selecting the correct testing protocol and improving future anode designs.
The Accelerated Life Test (ALT): Methodology and Standards
Under normal industrial operating conditions, a high-quality titanium anode can last anywhere from 2 to 20 years. Testing for such a duration in a laboratory setting is impractical. To solve this, engineers utilize the Accelerated Life Test (ALT), a method designed to compress years of wear into hours or days by intensifying the stress factors on the electrode.
The core principle of ALT involves increasing the current density significantly beyond the designed operational limit. This accelerates the electrochemical reactions and coating degradation while maintaining the same fundamental failure mechanisms.
Standard Testing Protocols:Most manufacturers and research institutions follow variations of the HG/T 2471 or NACE standards. A typical ALT setup includes:
- High Current Density: Often ranging from 10,000 A/m2 to 20,000 A/m2, compared to standard industrial loads of 200–1000 A/m2.
- Standardized Electrolyte: Usually a 1.0 M H2SO4 solution for oxygen evolution anodes or concentrated NaCl for chlorine evolution anodes.
- Controlled Temperature: Maintained at a constant level (e.g., 40°C ± 5°C) to ensure consistency.
The test continues until the cell voltage increases by 10 V (or a specific threshold) from the initial stable operating voltage. This "sudden jump" in voltage indicates the total failure of the anode coating and the onset of substrate passivation. By recording the time to failure (in hours), engineers can calculate the Enhanced Life Value of the coating.
Key Parameters in ALT: Current Density and Electrolyte Concentration
The reliability of an Accelerated Life Test depends on how strictly the experimental parameters are controlled. If the stress factors are too extreme, the failure mechanism might change, rendering the results useless for real-world prediction. If they are too low, the test becomes inefficient.
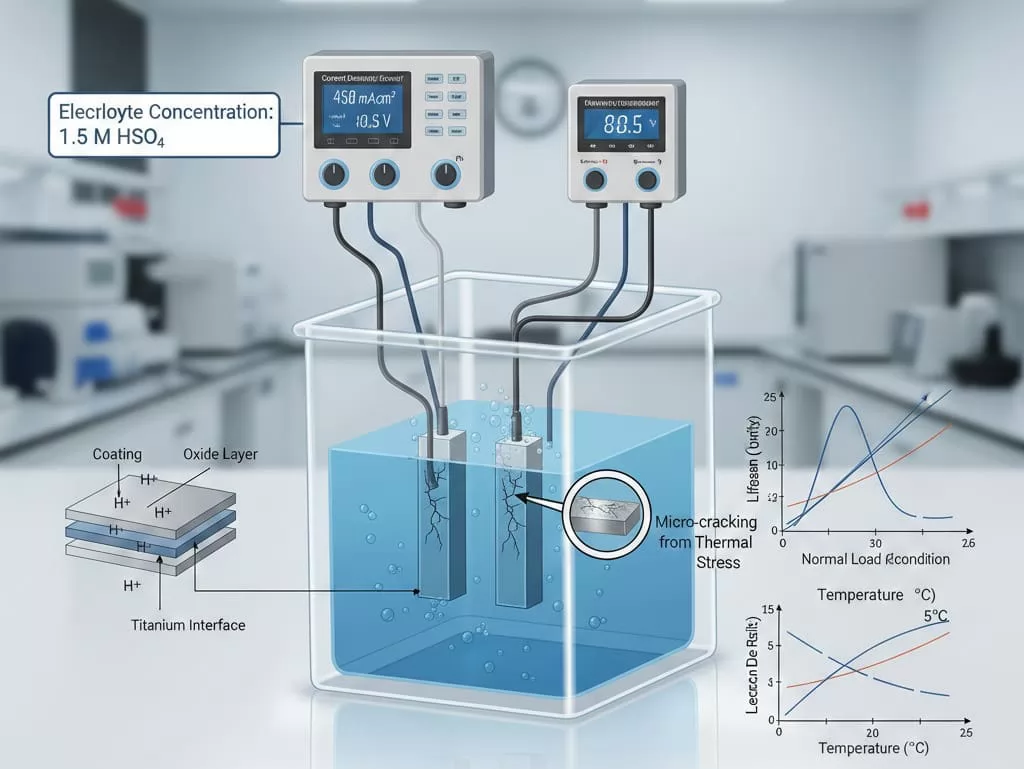
1. Current Density (j)Current density is the primary driver of coating degradation. In a standard ALT, the current density is typically set 10 to 50 times higher than the actual field application.
- High Load Impact: Excessive current can cause thermal stress, leading to micro-cracking of the oxide layer that might not occur under normal loads.
- Uniformity: Ensuring a uniform current distribution across the test sample is critical to avoid premature edge failure.
2. Electrolyte Concentration and CompositionThe chemical environment significantly affects the overpotential. For instance, in H2SO4 solutions, the focus is on oxygen evolution resistance.
- Acid Concentration: Typically 1.0 M to 2.0 M. Higher concentrations accelerate the penetration of the coating by H+ ions, reaching the titanium interface faster.
- Conductivity: The electrolyte must have high conductivity to prevent excessive ohmic heating during the test.
3. Temperature ControlElectrochemical reaction rates are temperature-dependent. A fluctuation of even 5°C can significantly alter the lifespan results. Most laboratory protocols require a water bath or cooling system to maintain a stable temperature, preventing the heat generated by high-current electrolysis from skewing the data.
Visual and Microscopic Analysis: Monitoring Surface Morphology Changes
While voltage data provides a quantitative measure of failure, surface morphology analysis offers a qualitative look at why the anode is degrading. By examining the physical state of the coating at different intervals during the testing process, engineers can identify structural weaknesses.
1. SEM (Scanning Electron Microscopy) ObservationSEM is the industry standard for inspecting the "mud-crack" structure typical of Thermal Decomposition coatings.
- Early Stage: The cracks are narrow and the coating grains are tightly packed.
- Mid-Test: Cracks widen and deepen, allowing the electrolyte to reach the titanium substrate.
- End of Life: Significant thinning of the coating is visible, with large areas of the titanium base showing signs of oxidation (passivation).
2. EDS (Energy Dispersive Spectroscopy)Used in conjunction with SEM, EDS allows technicians to map the elemental distribution on the surface. By tracking the ratio of Iridium (Ir) or Ruthenium (Ru) to the Titanium (Ti) substrate over time, we can calculate the consumption rate of the active precious metals.
3. Macroscopic InspectionBefore using high-tech instruments, simple visual checks are vital.
- Color Changes: A shift from dark black/grey to a lighter or yellowish tint often indicates the depletion of metal oxides and the growth of TiO2.
- Peeling/Spalling: Large-scale detachment of the coating suggests poor adhesion or excessive gas pressure within the coating pores.
By combining visual inspection with electrochemical data, manufacturers can determine if a failure was due to chemical dissolution (thinning) or mechanical failure (delamination), leading to better coating formulations.
Voltage-Time Curves: Identifying the Onset of Anode Passivation
The most critical data point in lifespan testing is the Voltage-Time (V-t) curve. During an Accelerated Life Test, the cell voltage is recorded continuously. This curve typically follows a three-stage progression that serves as a "DNA profile" for the anode's health.
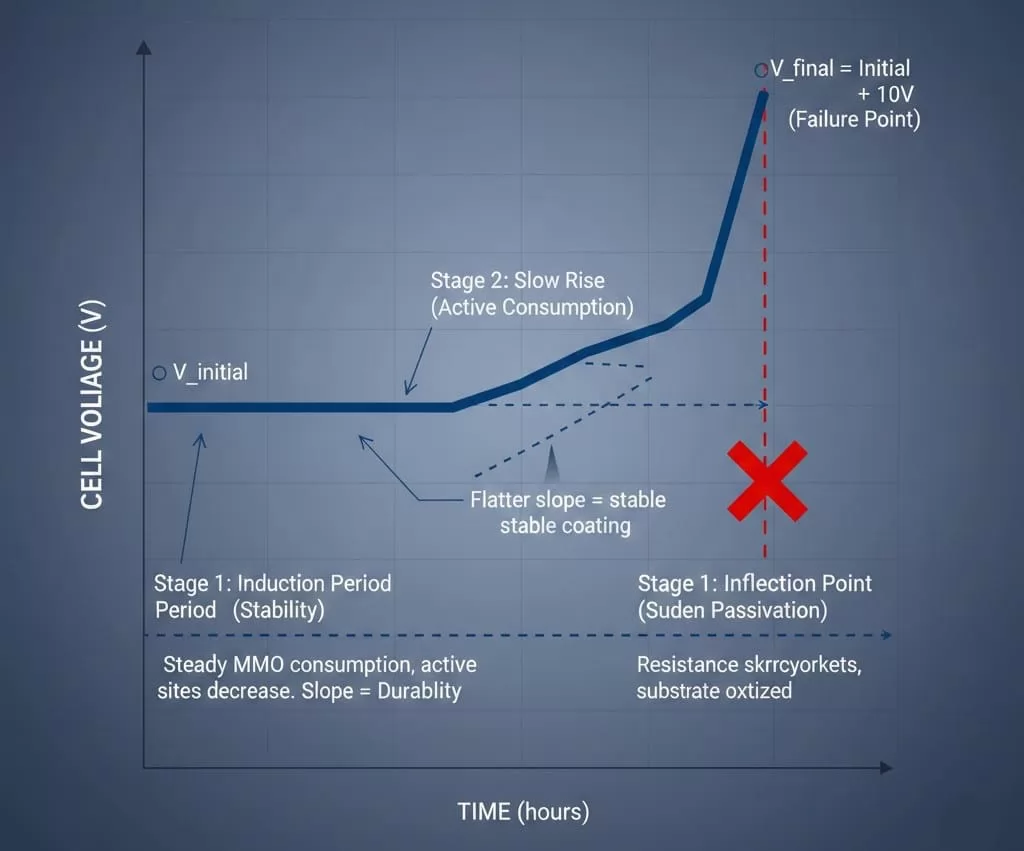
Stage 1: The Induction Period (Stability)Initially, the voltage remains relatively flat. The coating is active, and the electrochemical surface area is at its maximum. Any slight initial drop in voltage is usually due to the electrolyte reaching its optimal operating temperature and the full wetting of the coating pores.
Stage 2: The Slow Rise (Active Consumption)As the test progresses, a gradual increase in voltage occurs. This represents the steady-state consumption of the Mixed Metal Oxide (MMO) layer. The overpotential increases as the number of active catalytic sites decreases.
Stage 3: The Inflection Point (Sudden Passivation)This is the "failure point." Once the coating is sufficiently depleted or the titanium substrate is exposed and oxidized, the resistance skyrockets. The curve turns sharply upward. In standard testing, an increase of 10 V over the initial baseline is defined as the end of the anode's life.
By analyzing the slope of Stage 2, engineers can determine the durability of the coating formulation. A flatter slope indicates a more stable coating with better resistance to dissolution, while a steep slope suggests rapid degradation that may require adjustment of the Ir/Ru ratios.
From Lab to Field: How to Extrapolate Real-World Service Life
The primary goal of an Accelerated Life Test (ALT) is to provide a predictive value for how many years an anode will last under actual working conditions. Because the test uses extreme current densities, we use mathematical models to translate "test hours" into "service years."
The Empirical RelationshipThe most widely used calculation in the industry is based on the inverse relationship between current density and time. The simplified formula is expressed as:
L1 ⋅ j1n = L2 ⋅ j2n
Where:
- L1: Actual service life in the field (hours/years).
- L2: Accelerated life measured in the lab (hours).
- j1: Actual operating current density (e.g., 500 A/m2).
- j2: Accelerated testing current density (e.g., 10,000 A/m2).
- n: The acceleration exponent (typically ranging from 1.5 to 2.0 depending on the coating type).
Example Calculation:If an anode lasts 100 hours at a test current of 20,000 A/m2, and the actual field current is 1,000 A/m2 (with n = 2): The extrapolated life would be 100 × (20,000 / 1,000)2 = 40,000 hours, or approximately 4.5 years of continuous operation.
It is important to note that these calculations provide a theoretical maximum. In practice, environmental factors like electrolyte purity and temperature fluctuations often introduce a "safety factor" of 0.7–0.8 to the final estimate to ensure conservative maintenance scheduling.
Factors Influencing Testing Accuracy: Temperature and Impurities
While the Accelerated Life Test (ALT) is a powerful tool, its accuracy depends on how well the laboratory environment mimics the stressors of the field. Several variables can skew the results, leading to either an overestimation or underestimation of the anode's true service life.

1. Electrolyte Purity and ImpuritiesThe presence of specific "contaminant" ions can drastically change the failure mode.
- Fluoride Ions (F−): Even in small concentrations, fluoride can attack the titanium substrate directly, causing the coating to peel off long before the active oxides are consumed.
- Organic Additives: In electroplating baths, organics can adsorb onto the coating surface, increasing the local overpotential and causing localized "hot spots" that accelerate wear.
- Manganese/Iron Scaling: These can form a physical scale on the anode, blocking the active sites and leading to localized passivation.
2. Operating TemperatureAs discussed earlier, heat is a catalyst for chemical reactions. In a lab setting, if the electrolyte is not cooled effectively during high-current testing, the ohmic heat can raise the temperature beyond the design limit. This often results in a lower recorded lifespan because the rate of TiO2 growth (passivation) increases exponentially with temperature.
3. Gas Evolution DynamicsAt extreme current densities, gas bubbles (O2 or Cl2) are generated much faster than in real-world applications. If the test setup doesn't allow for efficient gas release, a "gas film" can form on the anode surface. This increases the actual current density on the remaining wetted areas, leading to non-uniform wear and premature failure.
To ensure accuracy, it is best practice to perform testing using a sample of the actual industrial electrolyte whenever possible, rather than a pure laboratory acid solution.
Integrating Testing into Predictive Maintenance for Electrolytic Systems
For large-scale industrial operations, replacing titanium anodes is a significant capital expenditure. Integrating lifespan testing data into a broader predictive maintenance strategy allows facilities to optimize the total cost of ownership (TCO) and prevent system-wide failures.
1. Real-Time Voltage Monitoring By comparing real-time field data with the Voltage-Time curves generated during laboratory ALT, engineers can pinpoint exactly where an anode sits on its degradation timeline. If the field voltage begins to deviate from the predicted slope, it serves as an early warning of electrolyte contamination or abnormal operating conditions.
2. Planned Rotation and Re-coating Titanium is a valuable substrate. Life prediction data helps determine the "Re-coating Window."
- Early Recovery: If the anode is pulled before the titanium substrate passivates completely, the substrate can be cleaned and re-coated at a fraction of the cost of a new assembly.
- Late Failure: If the anode runs until the substrate is heavily pitted or deformed by high resistance, the entire unit may need to be scrapped.
3. Energy Consumption Optimization As the anode coating reaches its end of life, the cell voltage rises to maintain the same current density. This increase translates directly into higher electricity costs. Predictive maintenance models use lifespan data to calculate the "Economic Break-even Point"—the specific moment when the cost of extra electricity exceeds the cost of replacing the anodes.
Implementing a data-driven maintenance schedule ensures that every gram of precious metal coating is utilized efficiently without risking the integrity of the electrolytic cell.
Selecting the Correct Anode Coating to Maximize Operational Longevity
The ultimate goal of lifespan testing is to validate that the chosen anode is fit for its specific purpose. Not all titanium anodes are created equal; the chemical composition of the Mixed Metal Oxide (MMO) coating must be precisely matched to the primary electrochemical reaction of the application.
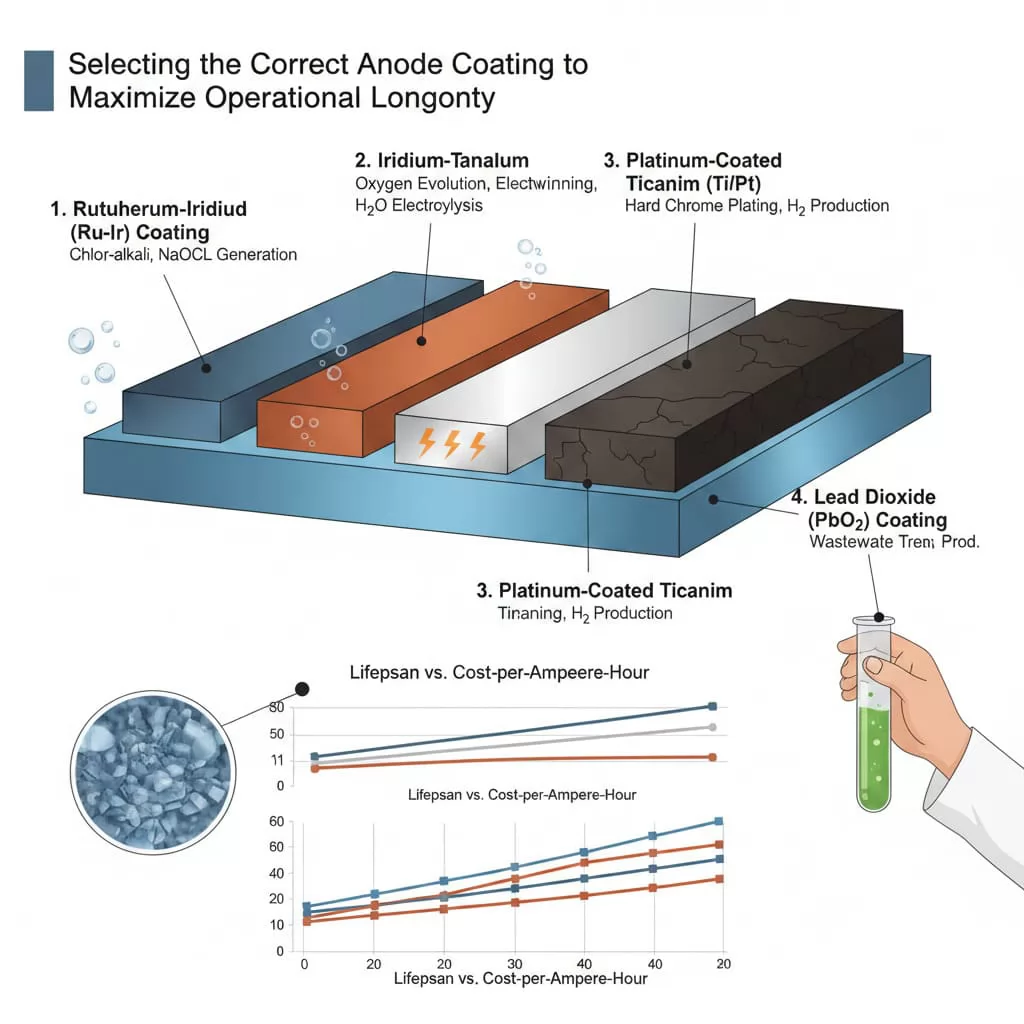
1. Ruthenium-Iridium (Ru-Ir) CoatingsThese are the industry standard for Chlor-alkali processes and sodium hypochlorite generation. They offer excellent catalytic activity for chlorine evolution. However, they are sensitive to oxygen evolution, which can accelerate coating consumption if the electrolyte pH or chloride concentration is not maintained.
2. Iridium-Tantalum (Ir-Ta) CoatingsEngineered specifically for Oxygen Evolution in acidic environments, such as electrowinning, electrogalvanizing, and water electrolysis. Ir-Ta anodes are highly resistant to acid corrosion and provide a much longer service life in environments where no chlorides are present.
3. Platinum-Coated Titanium (Ti/Pt)Often used in hard chrome plating or hydrogen production. While more expensive, platinum provides superior conductivity and stability in high-acid, high-current applications where MMO might fail prematurely due to substrate passivation.
4. Lead Dioxide (PbO2) CoatingsUsed for high-potential oxygen evolution reactions like wastewater treatment (COD removal) or perchlorate production. These anodes are characterized by their extreme hardness and thickness, though they require careful handling to prevent mechanical cracking.
By conducting thorough lifespan testing on different coating formulations, engineers can select the material that provides the best cost-per-ampere-hour, ensuring that the industrial process remains both productive and profitable over the long term.
About JH Ti Anode
Located in "China Titanium Valley" (Baoji), Shaanxi Jinhan Rare Precious Metal Co., Ltd. (JH) is a specialized manufacturer dedicated to the R&D and production of high-performance titanium anodes. Since 2009, we have integrated advanced materials science with precise manufacturing to support the global electrochemical industry.
Our core product range includes Ruthenium-Iridium, Iridium-Tantalum, Platinum, and Lead Dioxide anodes, alongside custom-engineered electrolytic systems. With a strong focus on reliability and technical innovation, JH has become a trusted partner for over 300 customers worldwide in sectors such as water treatment, electroplating, and hydrogen production.
Why Choose JH Ti Anode?
- Proven International Expertise: With over 80% of our products exported, we meet rigorous global standards and understand the diverse technical requirements of international markets.
- Decade of Innovation: Established in 2009, our 15+ years of experience ensure we deliver anodes with optimized corrosion resistance and superior electrolysis efficiency.
- Data-Driven Quality: Supported by a team of 60+ professionals, every batch undergoes strict quality control to ensure service life consistency.
- Scalable Solutions: From individual components to complete $8,000,000+ annual sales capacity, we provide both standardized products and customized technical systems.
Contact Our Engineering Team
Need a precise lifespan estimate for your specific electrolytic environment? Contact the JH technical team today for a consultation on anode selection and custom coating designs.
-
 Oct 29, 2025What is a Titanium Anode (Complete Guide)
Oct 29, 2025What is a Titanium Anode (Complete Guide) -
 Dec 19, 2025Titanium Anode Lifespan Testing: How to Accurately Predict Service Life
Dec 19, 2025Titanium Anode Lifespan Testing: How to Accurately Predict Service Life -
 Dec 25, 2025Titanium Anodes vs Graphite Anodes: Service Life and Cost Analysis
Dec 25, 2025Titanium Anodes vs Graphite Anodes: Service Life and Cost Analysis -
 Dec 17, 2025MMO Coated Titanium Anodes vs Traditional Anodes: Complete Comparison
Dec 17, 2025MMO Coated Titanium Anodes vs Traditional Anodes: Complete Comparison
-
 Jan 22, 2026PCB Circuit Board Plating: Titanium Anode Applications in Practice
Jan 22, 2026PCB Circuit Board Plating: Titanium Anode Applications in Practice -
 Jan 13, 2026Automotive Parts Plating: How Titanium Anodes Significantly Enhance Coating Quality and Performance
Jan 13, 2026Automotive Parts Plating: How Titanium Anodes Significantly Enhance Coating Quality and Performance -
 Jan 05, 2026Electrochemical Solutions for Hospital Wastewater Treatment
Jan 05, 2026Electrochemical Solutions for Hospital Wastewater Treatment -
 Dec 25, 2025Titanium Anodes vs Graphite Anodes: Service Life and Cost Analysis
Dec 25, 2025Titanium Anodes vs Graphite Anodes: Service Life and Cost Analysis
