Titanium Anodes vs Graphite Anodes: Service Life and Cost Analysis
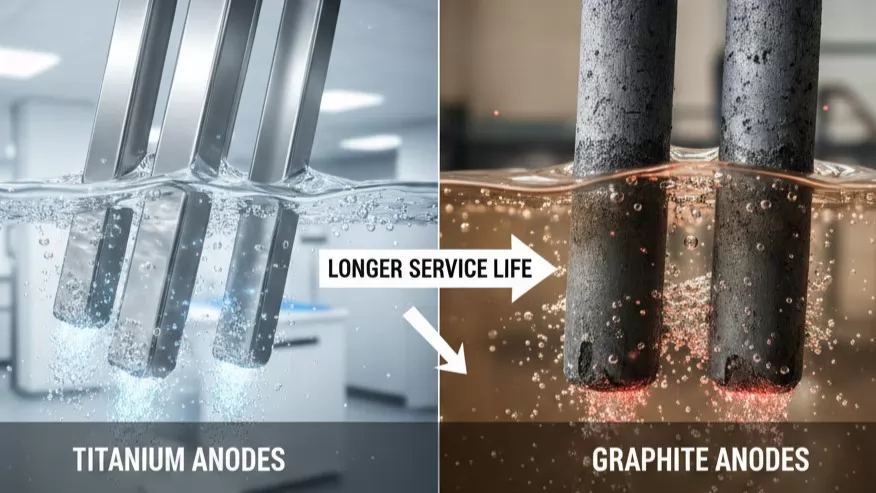
Table of Contents
- Introduction: The Technological Shift from Graphite to Titanium
- Material Composition: Substrate Properties and Coating Chemistry
- Electrochemical Efficiency: Oxygen and Chlorine Evolution Overpotential
- Dimensional Stability: Why Geometric Integrity Matters in Electrolysis
- Service Life Comparison: Durability in Corrosive Environments
- Energy Consumption Analysis: Voltage Efficiency and Power Savings
- Maintenance Requirements and Reducing Operational Downtime
- Total Cost of Ownership (TCO): Initial Investment vs. Long-term ROI
- Application Suitability: Selecting the Right Anode for Your Industry
- Environmental Impact: Waste Reduction and Sustainability Factors
Introduction: The Technological Shift from Graphite to Titanium
For decades, graphite anodes served as the primary choice for industrial electrochemical processes, including chlor-alkali production and metal electrowinning. Their widespread adoption was largely due to their low initial material cost and acceptable electrical conductivity. However, as industrial requirements for energy efficiency and product purity became more stringent, the inherent mechanical and electrochemical limitations of graphite began to pose significant operational challenges.
The development of titanium-based Dimensionally Stable Anodes (DSA) represented a fundamental shift in electrochemical engineering. Unlike graphite electrodes, which are "consumable" and physically degrade during electrolysis, titanium anodes consist of a rigid titanium substrate coated with electrocatalytic noble metal oxides (such as Ruthenium-Iridium or Iridium-Tantalum). This transition has allowed plants to move from high-maintenance, fluctuating systems to stable, high-performance environments.
Modern industrial facilities now prioritize the Total Cost of Ownership (TCO) over the initial purchase price. While titanium anodes require a higher upfront investment, their ability to maintain constant geometric dimensions and lower operating voltages offers long-term financial advantages. This analysis examines the technical distinctions that make titanium the superior choice for modern high-intensity electrochemical applications.
Material Composition: Substrate Properties and Coating Chemistry
The fundamental performance gap between these two materials begins at the molecular level. Graphite anodes are manufactured from high-purity petroleum coke and coal tar pitch, which are pressed and baked. While conductive, the resulting structure is inherently porous and brittle. During electrolysis, the graphite lattice is susceptible to mechanical erosion and chemical oxidation, leading to a "consumable" electrode that loses mass over time.
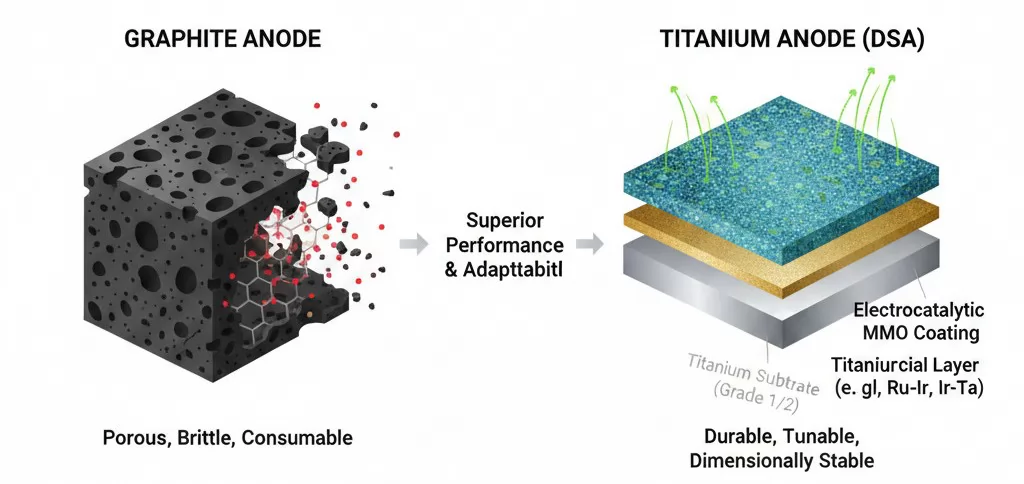
In contrast, Titanium Anodes (Dimensionally Stable Anodes or DSA) utilize a multi-layered composite structure designed for durability and specific catalytic activity:
- The Titanium Substrate: Typically Grade 1 or Grade 2 Titanium. This base provides exceptional corrosion resistance in harsh electrolytes and serves as a rigid mechanical support that does not deform under heat or pressure.
- The Electrocatalytic Coating: The substrate is coated with Mixed Metal Oxides (MMO) such as Ruthenium-Iridium (Ru-Ir), Iridium-Tantalum (Ir-Ta), or Platinum (Pt). These noble metal oxides are the active sites for electrochemical reactions.
- The Interfacial Layer: A specialized thin layer between the substrate and coating that prevents the formation of non-conductive titanium dioxide (TiO2), ensuring long-term electrical conductivity.
This combination allows for the fine-tuning of the anode's properties. For instance, Ru-Ir coatings are optimized for chlorine evolution in brine electrolysis, while Ir-Ta coatings are engineered for oxygen evolution in highly acidic environments. Graphite lacks this adaptability; its chemical performance is fixed by its carbon structure, offering no opportunity for application-specific optimization.
Electrochemical Efficiency: Oxygen and Chlorine Evolution Overpotential
In industrial electrochemistry, efficiency is largely defined by "overpotential"—the extra energy required to drive a chemical reaction beyond its thermodynamic equilibrium. A lower overpotential directly translates to lower energy consumption and higher throughput. Titanium anodes, through their engineered Mixed Metal Oxide (MMO) coatings, offer significantly lower overpotential compared to traditional graphite electrodes.
The performance gap is most evident in two primary reactions:
- Chlorine Evolution: In brine electrolysis, Ruthenium-Iridium coated titanium anodes exhibit a much lower chlorine evolution potential. Graphite anodes require higher voltages to achieve the same current density, leading to wasted energy dissipated as heat.
- Oxygen Evolution: For processes like electrowinning or water treatment, Iridium-Tantalum coatings provide a stable, low-overpotential path for oxygen evolution. Graphite, under the same conditions, undergoes rapid electrochemical oxidation (CO2 formation), which consumes the anode itself while requiring higher voltage.
Because titanium anodes maintain a consistent catalytic surface, the current efficiency remains stable throughout the production cycle. Graphite, however, suffers from "sloughing"—the shedding of carbon particles—which increases the inter-electrode gap and further degrades electrical efficiency over time. By switching to titanium, facilities typically see a measurable reduction in cell voltage, often ranging from 150mV to 500mV depending on the application.
Dimensional Stability: Why Geometric Integrity Matters in Electrolysis
One of the most critical advantages of titanium anodes is their name-sake property: they are Dimensionally Stable Anodes (DSA). In any electrochemical cell, the distance between the anode and the cathode (the inter-electrode gap) directly dictates the electrical resistance. A stable gap ensures a predictable and uniform electric field across the entire surface of the electrode.
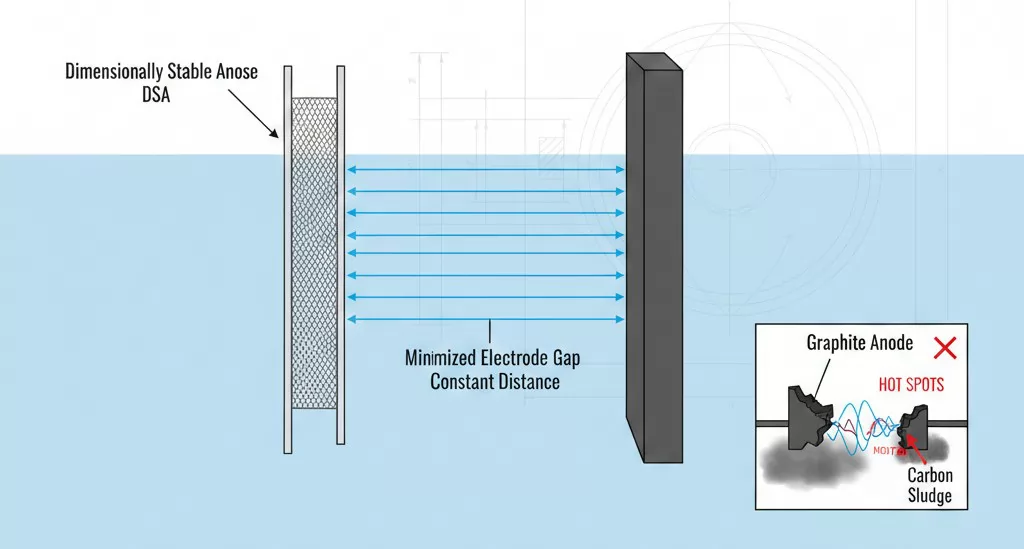
The geometric integrity of the anode influences the process in several key ways:
- Constant Voltage: Because the titanium substrate does not dissolve or erode, the distance between electrodes remains constant. This prevents the "voltage creep" typically seen with graphite as it wears thin.
- Uniform Current Distribution: A rigid, non-deforming titanium mesh or plate ensures that current density is even. In graphite systems, uneven wear leads to "hot spots," which can damage the cathode or degrade the quality of the final product.
- Product Purity: As graphite anodes degrade, carbon "sludge" or particles contaminate the electrolyte and the finished product (such as plated metals or chlorine gas). Titanium anodes eliminate this source of contamination, which is vital for high-purity medical or electronic applications.
For engineers, dimensional stability means the cell can be designed with a minimized electrode gap to optimize power efficiency without the risk of short-circuiting or mechanical interference. This allows for more compact cell designs and higher production throughput within the same physical footprint.
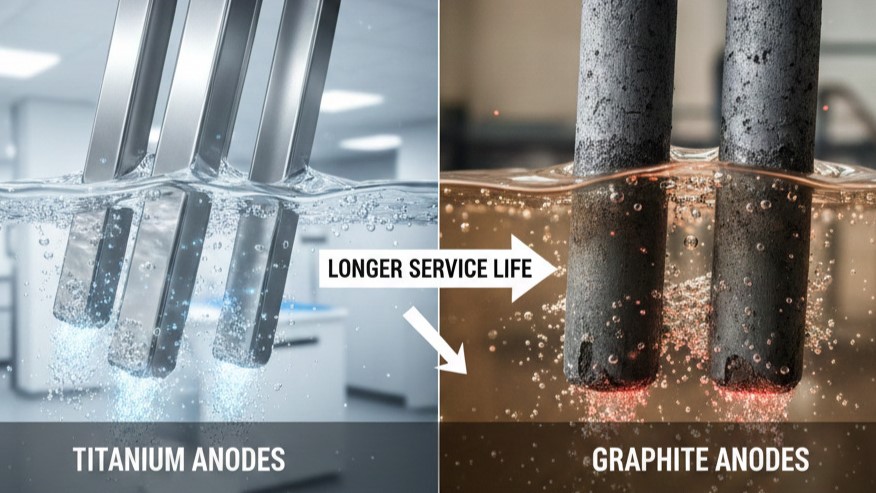
Service Life Comparison: Durability in Corrosive Environments
The most significant operational difference between graphite and titanium anodes is their lifespan. In industrial environments, service life is not just a measure of time, but a measure of process stability and cost-efficiency. Graphite is a "consumable" material, meaning it is expected to degrade and eventually disappear during the electrolysis process. Titanium anodes, however, are designed for multi-year durability.
The lifespan of these materials varies based on the chemical environment:
- Chlor-Alkali Industry: Graphite anodes typically last between 6 to 12 months before the structural thinning necessitates replacement. High-performance Ru-Ir coated titanium anodes in the same environment often exceed 5 to 8 years of continuous service.
- Electroplating and Electrowinning: In acidic sulfate baths, graphite can fail within weeks due to rapid oxidation. Ir-Ta coated titanium anodes are engineered to withstand these aggressive conditions, providing stable performance for 2 to 5 years depending on the current density.
- Water Treatment: In low-conductivity or high-chloride water applications, titanium anodes maintain their catalytic activity far longer than carbon-based alternatives, which tend to crumble and pollute the treated water.
A unique advantage of titanium is the substrate reusability. When the noble metal oxide coating on a titanium anode eventually wears thin (reaching its "end of life"), the titanium base remains intact. It can be stripped, cleaned, and re-coated—a process that is significantly more cost-effective than purchasing a brand-new electrode. Graphite provides no such option; once it is consumed, it must be completely replaced and the waste disposed of.
Energy Consumption Analysis: Voltage Efficiency and Power Savings
In large-scale industrial electrolysis, electricity is often the single largest operating expense, accounting for up to 70% of total production costs. The shift from graphite to titanium anodes is primarily driven by the significant reduction in specific energy consumption (kWh per ton of product). This efficiency gain is achieved through the combination of lower overpotential and a stable inter-electrode gap.
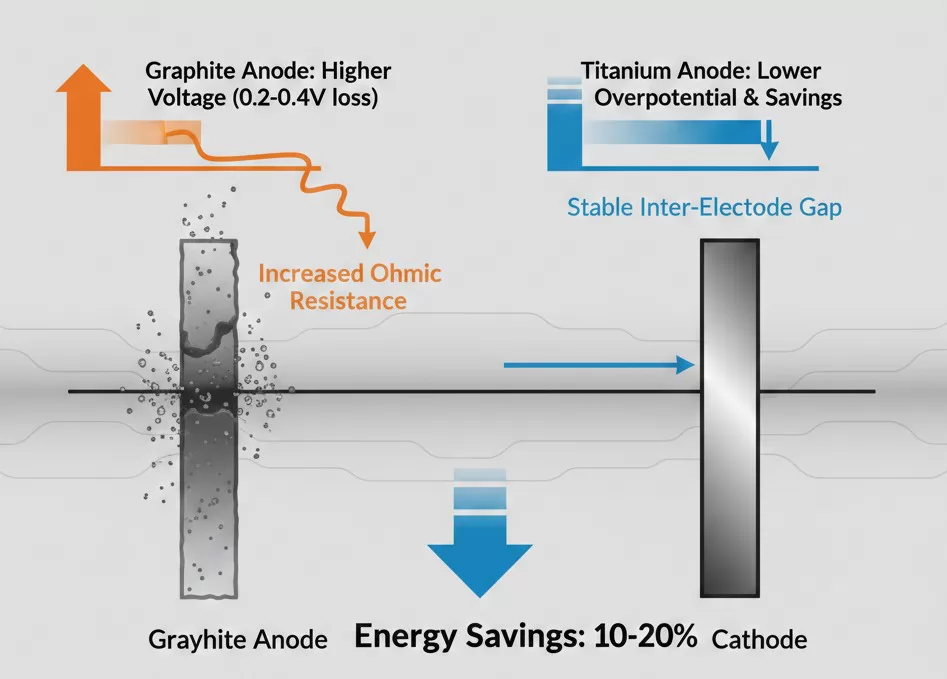
The total cell voltage ($V_{cell}$) is the sum of several components, where titanium anodes provide advantages in two critical areas:
- Reduction in Overpotential: Because the MMO coating is a more efficient catalyst than carbon, the activation energy required for the reaction is lower. This typically reduces the cell voltage by 0.2V to 0.4V compared to graphite.
- Ohmic Loss Control: Graphite anodes increase the electrolyte resistance as they wear down and the gap widens. Titanium’s dimensional stability keeps the ohmic resistance ($I times R$) at a minimum throughout the anode's entire life cycle.
To put this into perspective: for a medium-sized chlor-alkali or electroplating plant, a reduction of just 0.1V in cell voltage can result in tens of thousands of dollars in annual electricity savings. When moving from graphite to titanium, the cumulative reduction in voltage often leads to energy savings of 10% to 20%. This drastic reduction in power consumption often allows the equipment to pay for itself through energy savings alone within the first 12 to 18 months of operation.
Maintenance Requirements and Reducing Operational Downtime
In a high-capacity industrial facility, the cost of maintenance is not just the price of parts and labor—it is the lost revenue during downtime. Graphite anodes require a rigorous and frequent maintenance schedule. Because they are consumable, they must be periodically adjusted to compensate for thickness loss and eventually replaced entirely, requiring a full system shutdown.
The maintenance challenges associated with graphite include:
- Sludge Removal: As graphite wears down, carbon particles accumulate at the bottom of the electrolytic cell. This "sludge" must be regularly cleaned to prevent short circuits and electrolyte contamination.
- Frequent Handling: High replacement frequency increases the exposure of maintenance personnel to corrosive chemicals and heavy lifting, raising safety risks.
- Re-leveling: Engineers must frequently re-level and adjust graphite electrodes to maintain current efficiency as the material thins.
Titanium anodes offer a "set it and forget it" advantage. Once installed, they require minimal intervention. There is no carbon debris to clean, and the electrode gap remains fixed, eliminating the need for manual adjustments. For a plant operator, this means switching from monthly or quarterly maintenance cycles to multi-year intervals. The resulting increase in Plant Availability significantly boosts the overall annual production capacity without adding capital equipment.
Total Cost of Ownership (TCO): Initial Investment vs. Long-term ROI
When comparing titanium anodes to graphite, a common hurdle for procurement departments is the "sticker shock" of the initial purchase. Titanium anodes, utilizing precious metal coatings like Iridium and Ruthenium, have a significantly higher upfront cost. However, a technical Total Cost of Ownership (TCO) analysis reveals that titanium is the more economical choice over a 3-to-5-year horizon.
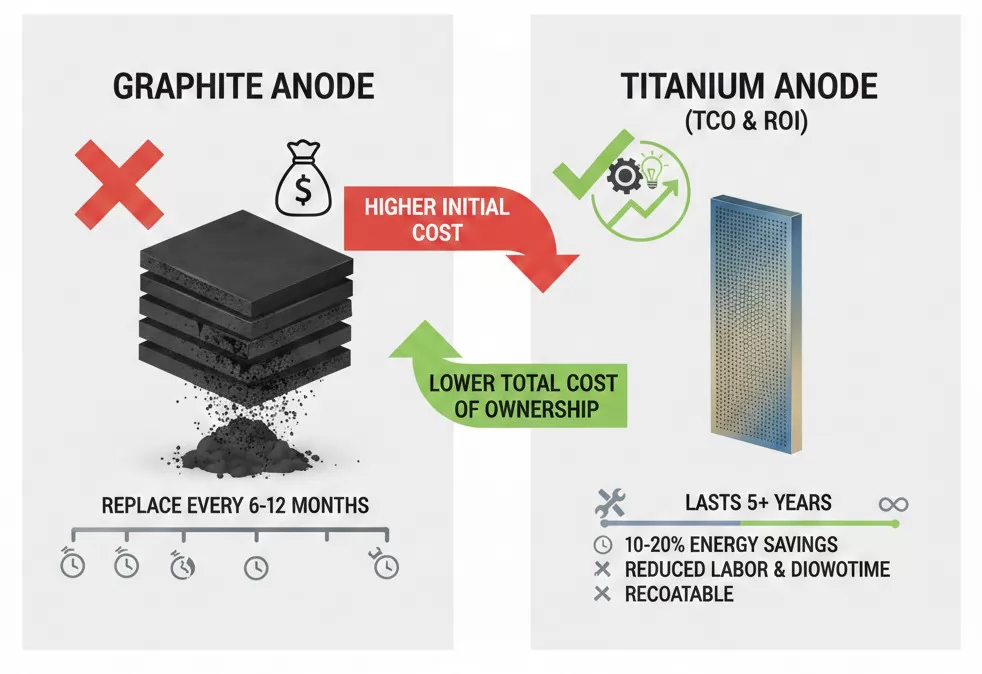
The TCO is calculated by summing the following factors:
- Initial CAPEX: Graphite is inexpensive; Titanium is a premium investment.
- Energy Expenses (OPEX): Titanium reduces electricity bills by 10–20% due to lower overpotential. In high-current applications, this saving alone often exceeds the cost of the anode within 18 months.
- Labor & Downtime: Graphite requires frequent replacement (every 6–12 months) and cleaning. Titanium lasts 5+ years, drastically reducing labor costs and lost production revenue.
- Residual Value: Graphite is a 100% loss once consumed. Titanium substrates can be re-coated at approximately 50–60% of the cost of a new anode, extending the asset's life indefinitely.
For engineers and financial controllers, the Return on Investment (ROI) of switching to titanium anodes is typically realized through cumulative power savings and the elimination of 4 to 10 replacement cycles that would have been required with graphite. When all variables are accounted for, the cost per ton of product is consistently lower with titanium technology.
Application Suitability: Selecting the Right Anode for Your Industry
While titanium anodes offer clear technical superiority, the choice of specific coating chemistry depends entirely on the industrial application. Unlike graphite, which is a "one-size-fits-all" but inefficient material, titanium anodes can be engineered to excel in specific chemical environments.
Below are the primary applications where the shift from graphite to titanium has become the industry standard:
- Chlor-Alkali & Hypochlorite Generation: Using Ruthenium-Iridium (Ru-Ir) coatings. Titanium anodes are essential here to prevent carbon contamination of the chlorine gas and to maintain the high efficiency required for mass production.
- Electroplating (Copper, Nickel, Chrome): Titanium anodes (often Platinum-coated or Ir-Ta coated) ensure a uniform plating thickness and high-quality surface finish that graphite cannot match due to its physical erosion.
- Water Treatment & Disinfection: For swimming pool electrolysis or industrial wastewater, titanium anodes provide long-term stability without introducing carbon debris into the water stream.
- Electrowinning: In the recovery of metals like Copper, Zinc, or Cobalt, Iridium-Tantalum (Ir-Ta) anodes offer the necessary acid resistance to survive in harsh sulfuric acid electrolytes where graphite would disintegrate rapidly.
For engineering teams, the transition involves evaluating the electrolyte composition, current density, and temperature. While graphite may still be found in legacy systems or low-budget, short-term laboratory setups, almost all modern industrial-scale operations have migrated to titanium to meet quality and efficiency benchmarks.
Environmental Impact: Waste Reduction and Sustainability Factors
In the modern industrial landscape, sustainability is no longer optional. The environmental footprint of an electrochemical plant is heavily influenced by its choice of electrodes. Graphite anodes, due to their consumable nature, present several ecological challenges that titanium anodes effectively mitigate.
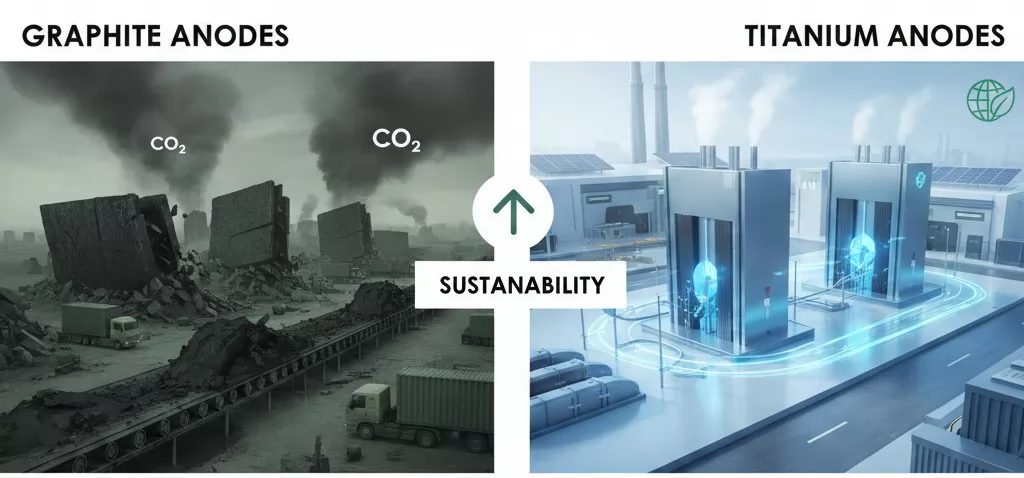
The environmental advantages of Titanium Anodes are centered around three core pillars:
- Waste Elimination: Graphite anodes continuously shed carbon particles and eventually become hazardous solid waste that requires specialized disposal. Titanium anodes are non-consumable; the substrate lasts for decades, significantly reducing the volume of industrial waste.
- Lower Carbon Footprint: Because titanium anodes operate at lower voltages, they consume less electricity. Since power generation is a primary source of CO2 emissions, the energy efficiency of titanium technology directly contributes to a smaller corporate carbon footprint.
- Reduced Logistics Impact: Replacing graphite every 6 months requires constant shipping, handling, and packaging. A titanium anode that lasts 5–10 years drastically reduces the environmental costs associated with the global supply chain and transport logistics.
Furthermore, the elimination of carbon "sludge" means that electrolyte solutions remain cleaner for longer periods. This reduces the frequency of electrolyte disposal and the need for intensive chemical filtration, leading to a more "closed-loop" and eco-friendly manufacturing process.
Company Introduction: Shaanxi Jinhan Rare Precious Metal Co., Ltd.
Located in Baoji, the world-renowned “China Titanium Valley,” Shaanxi Jinhan Rare Precious Metal Co., Ltd. (JH) is a premier high-tech enterprise specializing in the R&D and production of high-performance titanium anodes. With over a decade of expertise since our founding in 2009, we have established ourselves as a trusted global leader in electrochemical materials.
Our core product line includes Ruthenium-Iridium, Iridium-Tantalum, Platinum, and Lead Dioxide anodes, alongside customized electrolytic systems designed for electroplating, water treatment, and hydrogen production. We bridge the gap between material science and industrial application, transforming from a product supplier into a comprehensive solution provider.
Why Choose JH Ti Anode?
- Proven International Trust: With an 80%+ export ratio, our products meet the rigorous quality standards of 300+ global customers across the Americas, Europe, and Southeast Asia.
- Engineering Excellence: Our team of 60+ professionals focuses on improving corrosion resistance and electrolysis efficiency, backed by an annual sales volume exceeding USD 8,000,000.
- Innovation-Driven: We don’t just sell anodes; we design complete technical systems and customized electrolytic cells tailored to your specific chemical environment.
Contact Our Engineering Team
Ready to optimize your electrochemical process and reduce energy costs? Our technical team is available to provide detailed ROI analysis and coating recommendations for your specific application.
-
 Oct 29, 2025What is a Titanium Anode (Complete Guide)
Oct 29, 2025What is a Titanium Anode (Complete Guide) -
 Dec 19, 2025Titanium Anode Lifespan Testing: How to Accurately Predict Service Life
Dec 19, 2025Titanium Anode Lifespan Testing: How to Accurately Predict Service Life -
 Dec 25, 2025Titanium Anodes vs Graphite Anodes: Service Life and Cost Analysis
Dec 25, 2025Titanium Anodes vs Graphite Anodes: Service Life and Cost Analysis -
 Dec 17, 2025MMO Coated Titanium Anodes vs Traditional Anodes: Complete Comparison
Dec 17, 2025MMO Coated Titanium Anodes vs Traditional Anodes: Complete Comparison
-
 Jan 22, 2026PCB Circuit Board Plating: Titanium Anode Applications in Practice
Jan 22, 2026PCB Circuit Board Plating: Titanium Anode Applications in Practice -
 Jan 13, 2026Automotive Parts Plating: How Titanium Anodes Significantly Enhance Coating Quality and Performance
Jan 13, 2026Automotive Parts Plating: How Titanium Anodes Significantly Enhance Coating Quality and Performance -
 Jan 05, 2026Electrochemical Solutions for Hospital Wastewater Treatment
Jan 05, 2026Electrochemical Solutions for Hospital Wastewater Treatment -
 Dec 25, 2025Titanium Anodes vs Graphite Anodes: Service Life and Cost Analysis
Dec 25, 2025Titanium Anodes vs Graphite Anodes: Service Life and Cost Analysis
